ScooterTDI
BB Participant-
Posts
242 -
Joined
-
Last visited
Content Type
Profiles
Forums
Gallery
Events
Store
Everything posted by ScooterTDI
-
-
-
Thanks everyone! This has been a really fun project. I can't wait to see them develop adult coloration, but that may take a while.
-
They have definitely settled now and I still have 11. I didn't know they would get vertical striping. These guys really gobble up the Parvocalanus. I'm not sure when they'll be ready to transition to other foods.
-
As of 10 dph, I believe I am seeing settlement behavior in a few of the larva. A few are now swimming horizontally near surfaces whereas before they typically were oriented vertically and floating around with the current. The seemingly settled individuals also frequently adopt an "S" shape. I think 2 are too weak to make it more than a couple more days, but the other 10 are growing pretty well.
-
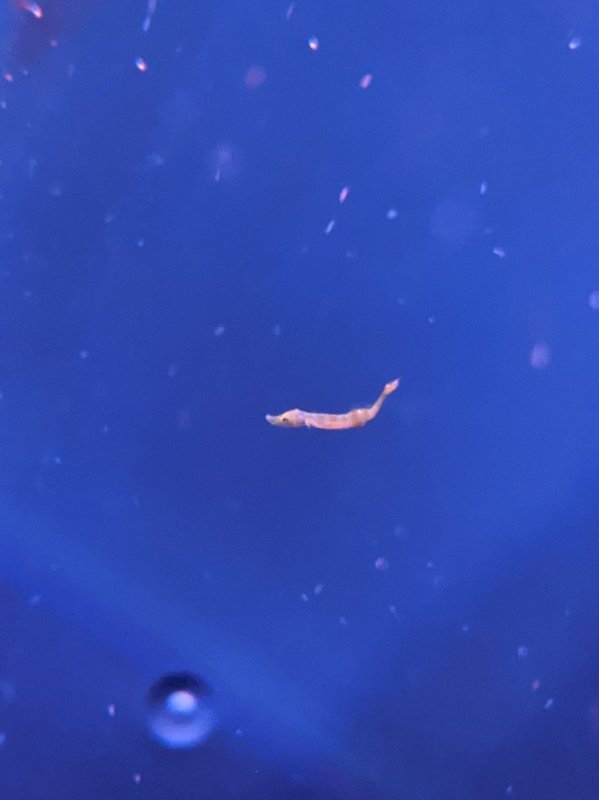
Breeding bluestripe pipefish has been a goal of mine for years, but I haven't made a serious effort in quite a while. Around 2009, I attempted to raise them on rotifers and artemia based on a German report that they could be raised without copepods, but I suspect they may have had some copepod contamination of their rotifer cultures. I never once saw the larva ingest rotifers or artemia and it seemed all died of starvation by day 3. By 2013, Parvocalanus were more available and so I tried to acquired broodstock for another breeding attempt, but all of the wild stock I received over several online orders was either DOA or in very poor health, so I gave up. Now, I have two healthy breeding pairs and appropriate copepod cultures, so I'm giving them another try. I currently have about a dozen at 9 days post-hatch and haven't lost any since about day 3, but there are a few that are clearly weaker and developing more slowly. The first picture is 5 dph and the second is 9 dph.
-
ScooterTDI's 12g Long Peninsula
ScooterTDI replied to ScooterTDI's topic in Dedicated Tank (Build) Forum
I got tired of occasionally tripping the breaker due to high startup current of power tools in the garage, so I ran a dedicated 20A circuit for the fish closet yesterday! -
ScooterTDI's 12g Long Peninsula
ScooterTDI replied to ScooterTDI's topic in Dedicated Tank (Build) Forum
So, I made a few changes to the fish closet lately. I'm spooling up breeding efforts for blue-striped pipefish, so I set up T-iso and Tetraselmis cultures as well as a couple of pretty dense Apocyclops panamensis cultures. I installed a shelf on the right side of the closet to support a 5g kriesel tank that I made. I've been trying to establish breeding pairs of blue-striped pipefish over the last couple months and now have two mated pairs. I'll see if I can get by with Apocyclops, but I may have to switch to Parvocalanus. -
Re-thinking the "grow out and trade" practice
ScooterTDI replied to treesprite's topic in General Discussion
Having seen giant schools of tangs in the wild roam over miles of reef, it doesn't really matter to me whether one is in a 4' or 5' tank. All of our aquariums are too small for a tang to really exhibit wild behaviors. That being said, I think buying a captive bred yellow tang would help to mitigate the moral quandary since it never has seen a wild reef and never will. -
I see what you did there. Name checks out.
-
One thing that I didn't bother doing for that project, but would be critical for use as an alkalinity monitor, is train the algorithm to determine the quantity of titrant added. That would allow the use of low precision pumps and negate the need for pump calibration. For this project, I was manually pipetting titrant, so I just used the known titrant volume as part of training.
-
I recently picked up IO at $25/bucket with a recurring purchase discount on Amazon. Not sure if the price will increase for next delivery though.
-
I couple years ago I researched, designed, and built a proof-of-concept prototype of an automated alkalinity monitor for a class I was taking in introductory machine learning for applications within chemical engineering. For a while I was thinking about trying to bring the design approach to market, but I've been tied up with other aspects of life, so I thought I'd just share it with everyone. The design concept is based upon the tracer-monitored titration technique introduced by Martz et al. 2006. I think this approach has several distinct advantages over existing alkalinity monitor designs: 1) No probe calibration necessary because there are no pH probes 2) No pump calibration because titrant volume is measured spectrophotometrically 3) Precision pumps are not needed which reduces cost and may reduce maintenance (piezoelectric small-volume pumps seem pretty attractive for this application) 4) Single inexpensive reagent 5) Demonstrated superior accuracy using minimal training data This prototype lack the ancillary hardware that would make the system fully automated (e.g. a pump to sample the water and flush the flow cell, a magnetic stirrer for the flow cell, etc), but does demonstrated that the technique itself works quite well. This design uses a standard acid titrant (0.02N H2SO4) spiked with an indicator dye (bromocresol green), i.e. the tracer. The titration is conducted in a cuvette (or flow cell if automated) and is monitored by a multi-channel DIY spectrophotmeter. The spectrophotmeter is designed to be fairly inexpensive and consists of an RGB LED emitter and a spectral sensor with 6 bands across the visible spectrum (both obtained from SparkFun). Together, the emitter and sensor can make 18 individual measurements for each discrete titrant volume added (3 emission bands x 6 sensor bands = 18). The software supporting this hardware uses machine learning techniques (i.e. fancy term for regression analyses) and is trained on the spectral response of sample with known alkalinity during titration. To do this training, I took samples of my tank water and adjusted the alkalinity through addition of acid so that I had many individual samples with different alkalinity within the range of plausible values. This was my final project for the class and I didn't have a lot of time to do the training, so I ended up only recording the spectral response of 11 individual samples. From that very small dataset, I performed a cross-validation (train on 10 and measure on 1) to demonstrate that it is possible to get accuracy as good as +/-2.6% with an appropriate machine learning algorithm. I expect that to improve significantly with a larger training set. I've attached my paper if anyone is interested. Please don't fault me for how the paper was written. I had to comply with many specific requirements for the assignment, so there are a bunch of rather uninteresting results included. CHE6665_Final_Project_Weinrich.pdf
-
ScooterTDI's 12g Long Peninsula
ScooterTDI replied to ScooterTDI's topic in Dedicated Tank (Build) Forum
I just got the regular reefbar. I wanted to keep the aluminum cover that I made to fit between the lights. It is made out of flat bar stock attacked to some C-channel, so the strip light had to fit inside the C-channel. The regular reefbar was basically the only one I could find that was small enough. It's not super bright, but makes the overall light color a little bluer and is plenty bright for corals within 6". -
ScooterTDI's 12g Long Peninsula
ScooterTDI replied to ScooterTDI's topic in Dedicated Tank (Build) Forum
I haven't measured par, but I imagine it's pretty high intensity given that most corals are less than 6" away. I recently added a 21led reefbar to fill in the middle a bit. The corals that were really high up (Hawkins and purple stylo) weren't getting as much light down the center. I still think it looks as yellow as any coral can under white light. Nutrients haven't changed much. That picture was filtered through a thick layer of green algae on the glass, so that might be tinting it a bit. I cleaned the glass about a week ago, so maybe this picture is a little more clear. (Also attaching a cool picture I took the other day from the end just for fun) -
ScooterTDI's 12g Long Peninsula
ScooterTDI replied to ScooterTDI's topic in Dedicated Tank (Build) Forum
-
Low Alkalinity and slowly raising it...
ScooterTDI replied to YHSublime's topic in General Discussion
Totally normal. I've got a similar water volume and I'm dosing 120 ml of each per day and it seems to fluctuate a bit depending on availability of nutrients. -
Glad to see I'm not the only one then. Good point on the livestock additions. I'm the opposite when it comes to livestock though. When the tank is doing well, my anxiety about introducing new corals or fish increases because I don't want to disrupt anything when its doing well. In particular, anxiety about introducing coral pests often makes me refrain from picking up any new corals.
-
I find myself constantly trying to think of ways to improve my tank system even though I think the system is generally in good health. For instance, I currently keep looking at LED strip lights to fill a gap in lighting on the display even though growth in the display has been fine. I also keep thinking about calcium reactors, but luckily the price has kept me away so far. Sometimes, this obsession has resulted in notable improvements. For instance, I recently changed out two smaller circulation pumps for one slightly larger circulation pump and found the flow in the display to dramatically improve. I spent 2 years fussing with those two original pumps unnecessarily (constantly cleaning to keep up flow, semi-frequent replacements, etc.) and really wish I had just experimented earlier to find a better solution. Other than sunk costs, some changes are easily reversible (like changing out the pumps), but others can cause major damage (bleaching with a new light, alkalinity spike from tuning a new calcium reactor, etc.) How do you know when things are "good enough" and how to you keep yourself from messing up a good thing?
-
Refugiums: How do we export nutrients?
ScooterTDI replied to sethsolomon's topic in General Discussion
I made one. I don't like the design of most DIY waterfall ATS or the commercially available ATS. I've heard too many stories of: 1) spraying water out of the tank as algae start to grow and block the spray bar, 2) the drain clogging causing flooding, or 3) cleaning algae/salt creep off the inside of the unit. I also wanted a IP67 (or better) rated light due to concerns about the proximity of water. My scrubber is a horizontal trough-style ATS and resides entirely below the rim of the sump, so the likelihood of water spillage is very low. You can only light the screen from one side, but there is no glass/acrylic between the screen and the light, so there is no need to clean anything to maintain light transmission. Other than the light fixture, I just made it out of materials I had lying around the garage.- 13 replies
-
- turf scrubber
- algae reactor
-
(and 4 more)
Tagged with:
-
Refugiums: How do we export nutrients?
ScooterTDI replied to sethsolomon's topic in General Discussion
One thing to keep in mind is that the nutrient export needs of the tank may evolve over time. I have been running an algae scrubber (>12 hrs./day, reverse photoperiod) to reduce growth of GHA and bryopsis in the display and things were stable for a long time, but I wasn't regularly monitoring nutrients and ended up dealing with dinoflagellates after nutrients bottomed out. I responded by turning off the algae for a week or so. Although the nutrients are still pretty much immeasurable, the dinos disappeared within a few weeks and I now run a reduced photoperiod on the scrubber (only about 4 hrs/day) just to keep it alive if I decide I need more export in the future. The alkalinity consumption of the tank increased by about 30% in the few weeks after I reduced algae scrubber photoperiod, so I think the corals were being growth limited by low availability of nitrate/phosphate. As corals fill in, they do seem to exert a significant nutrient demand. ATS are really quite efficient once they get going. I have a very high fish load in a ~40g total water volume and could adequately control nutrients with a 4x6" screen lit from a single side with a 20 watt LED fixture.- 13 replies
-
- turf scrubber
- algae reactor
-
(and 4 more)
Tagged with:
-
I've found that temperature to be the primary driver of variability like you are experiencing. If the refractometer, tank water, and calibration solution are at different temperatures, you are likely to experience measurement errors. For instance, if your refractometer and calibration solution are colder than the tank water, then the tank water may initially appear lower salinity than it actually is. As the refractometer cools down the tank water on the slide, the salinity will appear to increase as the density of the water on the slide increases. Recalibrating and re measureing multiple times while the temperature is unsteady can cause the apparent salinity to jump all over the place. The refractometer are advertised as being automatically temperature corrected (ATC), but I think this is just relying on the mass of aluminum around the stage to act as a heat sink and requires enough time for the temperature of the sample to equilibrate with that of the refractometer and assumed that the refractometer won't change temperatures substantially when handling during measurement. These issues can be avoided by either 1) storing the calibration solution and refractometer at the same temperature as the tank ( much preferred) or 2) at least letting the calibration solution and sample water sit on the slide for long enough to equilibrate temperatures. I used to have lots of problems in the winter when I was mixing saltwater in an unheated laundry room. When mixing, the water, calibration solution, and refractometer would all be quite cold. Then I'd heat up the water to tank temps, but still measure with a cold calibration solution and refractometer. This yielded a lower salinity than obtained at mixing. Then I'd add some salt to adjust and bring the water upstairs and compare to the salinity of the tank and it would be totally off (and changing over time as the temperature of the refractometer changed.) Now, I just store everything near tank temperatures and the refractometer calibration holds very well over long periods.
-
Along for the ride! You might want to consider locating the sump in the laundry room and run the plumbing to it under the stairs. It would be much more convenient to access and the utility sink is super close for cleaning equipment.
-
Are those wrasses a "Sustainable Islands" (AKA SI) product or a "Sustainable Aquatics" (AKA SA) product? Sustainable Aquatics markets sustainably caught wild fish as "Sustainable Islands". As far as I know, those aren't considered or marketed as "captive-raised". On their website, they don't have any wrasses listed under SA, but do have some wrasses listed under SI. Edit: Upon further investigation, it seems that the SI fish probably can be considered tank-raised. Although the SA website refers to SI fish as wild-caught, they are collected at larval stage or just post-settlement, which can reasonably be considered "tank-raised". Edit again: Upon even more investigation I'm again skeptical about whether these SI fish can be credibly considered tank-raised. There are a number of rather large fish on the SI list and I think that is a bit suspicious. For example, I don't think believe that they raised a Naso tang from post-settlement size (probably ~1") to a size of 8-12". I'm not sure how many of these SI fish are much different from the typical wild-caught fish.
-
Algae barn also has CB inverts, but they are only sporadically available. I have their CB urchins and peppermint shrimp. I would get their CB snails, but they are only occasionally available.