-
Posts
3,408 -
Joined
-
Last visited
Content Type
Profiles
Forums
Gallery
Events
Store
Posts posted by mogurnda
-
-
I have Evergrows over my main reef, macroalgae/slug display tank, and two algae growout tanks. The quality of the assembly is quite nice, and they have held out for years without LEDs failing or measurable reduction in PAR. The S2 that I dropped in the sump did fail, but I don't think that was the manufacturer's fault.
-
1. Slow down.
2. Your tank is a 20 gallon, no? Condylactis, LTAs, and BTAs are likely to spread out and roast everything in a wide radius, once they are settled in and happy. This is assuming they don't move. IMO, corals and anemones don't mix in small tanks.
-
Fascinating.
I liken your description of #1 to our fat cells.
#2 brings up the question, do these slugs have discernible fat cells? If so, #1 would seem to make less sense unless there was some reason to favor two separate strategies for energy storage.
#3 sounds interesting.
#5 - I like your test approach.
If the chloroplasts were being retained to produce energy and they required energy to harvest and maintain, what would happen if they were plunged into prolonged darkeness? Would they expel (or consume) the chloroplasts since they become an energy sink? Would the chloroplasts simply die?
More great questions, Tom. I honestly have no idea how they store energy. Unlike mammals and insects, there is not an obvious fatty tissue, but I expect that there are fat cells somewhere. They also store sugar as glycogen. On the other hand, energy storage may be a whole body kind of thing. They tend to grow fast when food is plentiful, and shrink down to nothing when they starve. Another area I need to know more.
As far as the effects of darkness, starved slugs in the dark do not lose color or the ability to photosynthesize any faster than controls kept in the light. The idea seems to be that both groups are digesting the chloroplasts once they are starved. I do not know of any studies in which they were allowed to feed while in the dark.
-
Thanks, Tom. I feel like we have more loose ends than data, but I guess that's a good thing. You ask some very good questions.
Could there still be some variation in preferred diet in the region? The samples that you collected were collected off of Codium, so it's not terribly surprising that the chloroplasts were found to be from Codium.Entirely possible. We surveyed about 8 spots in the bay, and the only places we found the slugs also had Codium, but the proof would be in the DNA. Part of the plan for next summer is to take samples from more slugs at more sites.
Not finding a mix of different DNA, though, suggests a preference of the individuals in your sample, I guess.It could mean they prefer to eat Codium, or that they preferentially keep the chloroplasts from Codium. In my office tanks, the Elysia clarki spent almost all of their time face down in Bryopsis, but the one good sequence we got last spring was from Halimeda incrassata, suggesting that the slugs were sneaking off to eat Halimeda, or that Halimeda chloroplasts that they do eat last longer. Other people have shown that some species of choloplasts last longer in the slugs as well. More work to be done.
Hey, if they don't keep the chloroplasts for nutrition, what's the reason for keeping them intact? Is that a question you'll try to address?That's the $64 question. It is clear that the chloroplasts produce energy, but nobody has done the calculation to figure out what portion of their metabolism could be supplemented by photosynthesis. I'd love to be able to do the measurements and calculations for that, but not just yet.
They have to do something for the slug. There is a metabolic cost to segregating and storing the chloroplasts, so there has to be some advantage to make it worthwhile. The abundance of Elysia worldwide suggests that the lifestyle is advantageous. There are several viable hypotheses:
- They serve as an energy store. Although photosynthesis does not contribute a lot to the slugs' energy budget, the chloroplasts themselves may be burned for energy when times get hard.
- They help the slugs generate and store energy when times are good. Photosynthesis may not be enough to prevent starvation, but the little bit of extra energy may help the slugs increase their fat stores.
- Products of the chloroplasts enhance the production of eggs. Once they get going, these gals (actually, hermaphrodites) produce a lot of eggs, and nobody has tested the role of photosynthesis or photosynthetic products on reproductive success.
- Visual camouflage. What better way to match your food plant? Seems a very metabolically expensive way to be green, but that doesn't mean it's not the case.
- Chemical camouflage. This is my current favorite. Chloroplasts produce a lot of chemicals, and these could help the slug smell like their food. Based on other molluscs, I expect that their major predators are nudibranchs, which are essentially blind and hunt by smell. I am still working on ways to test this in the field and in the lab. The idea would go something like: a) what eats the slugs; b) does blocking photosynthesis in the slugs make it easier for predators to find them.
- Chemical defense. Similar to the above, but the chloroplasts make the slugs taste bad to predators.
As I said, option 5 is the hypothesis which has the fewest flaws, at least in my mind. At the moment, we are all digesting what we learned this summer, taking care of business, and starting to think about next year. With the molecular tools and the info from the surveys, we are in an excellent position for next year.
A few more photos for fun:
The group, surveying and having fun.

Scorpion, under UV

Normal light. One of the best things about coming home was not having to worry about scorpions or rattlesnakes on the way to the bathroom.

-
Of course we found slugs. As always seems to be the case, finding the first one is the hard part.
On a day without fieldwork or lab work, I got up at the usual time, had my coffee and got ready to go.

After a few hours sweeping back and forth over the shallows, I saw an odd color in the Codium. A slug, yaay! As you can see in the photo below, it's not easy to see the gals. On a clear morning, it's not so bad, but it can be a real challenge when the surge kicks up. There are at least three, maybe four slugs in the Codium below.

They went into the holding tanks for behavioral observation and later DNA sampling. Note the little Aplysia to her left. We had a few dozen ride in with some algae we collected.

We knocked one of them out, and snipped off a piece of parapodium.

The team extracted and amplified the DNA, I took some of the samples back across the border for sequencing.
After cleaning up the samples to get rid of enzymes and whatnot, Macrogen USA (conveniently located in Rockville) returned some very nice sequence.
Below is an alignment of a short stretch of the gene of interest (rbcL), for Elysia and two species of algae we collected. The arrows indicate the sites at which the slug sequence differs from Ulva. The DNA from the slug kleptoplasts is essentially identical to Codium. Maybe not surprising, since they are always found near Codium, and often nestled among its branches. Still, it needed to be confirmed. Along the way, we got the first DNA sequence for the local species, Codium simulans.
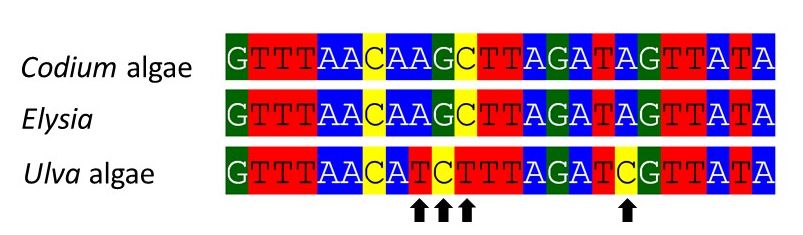
Short summary: the DNA work went better than we could have expected. We were up against a lot, but it all came together.
We still have a lot to do, like replicating the results in more individuals and curating the C. simulans samples, plus moving forward on behavioral work and field surveys. Next summer's work should be enough to get the initial DNA analysis published, and we can make a start on the other parts of the project.
-
Looked like we were ready to take it on the road. Originally, I had planned to grab some samples in Bahia de los Angeles (BLA), while working loosely with Ocean Discovery Institute, and do the extraction back in the lab at UMd. Somehow, we decided to make it a project for Ocean Discovery students to do the extraction and PCR in the field. That made it more interesting, but increased the chances of a train wreck. Well, what the heck.
There were quite a few sleepless nights, such as when it was not clear whether export or collecting permits would be available, and when the local company that was supposed to be building my holding tanks and acrylic “I-mazes” had still not delivered a week before I was supposed to leave. Fortunately, we got all the permissions, and I learned that Glass Cages is able to build and ship custom acrylic tanks at a moment’s notice. Anyway, after months of planning, buying equipment and reagents, and tracking down equipment that we could borrow and use in Mexico, we were off.
My students, “Dave’s Angels,” were great. Very enthusiastic, and very smart. I was also lucky to get Richy, a fellow from UCSB, to help out with logistics and with managing the students. You can see the Glasscages holding tanks through the windows at right.

We got the lab set up, and all was well, except that I found zero slugs in the first week. We could get started on the algae extractions, but would need slugs at some point.

Lots of good snorkeling, though, while we looked for Elysia.


The puffers by the station were too tame. They would eat anything you stirred up, which could mean the quick loss of a specimen.

As I said, Padina is abundant there, as is Sargassum.

Below is one of our survey sites at the island Gemelito Este.

Plus, we got to see the usual whales, dolphins and sea lions when we were out on the bay.

Will we find slugs? Stay tuned?
-
Time for an update from the spring and summer.
Very busy times. The goal for the summer was to determine which species of algae Elysia diomedea was eating at the field site in Bahia de los Angeles, Baja California. There are many reasons to study the little gals. For example, they graze in very shallow water, so are very dependent on specific conditions of clarity and nutrients. Also, they are kleptoplastic, meaning that they suck the sap out of their food algae, and digest everything but the chloroplasts. They keep the chloroplasts, which keep performing photosynthesis, and it was thought for many years that the slugs themselves became photosynthetic, like corals with their zooxanthellae. Turns out that is not true, so we have no idea why they steal chloroplasts.
Anyway, we can’t get very far if we don’t know what they eat. Based on our observations and those of others, we figured it was probably Codium, Bryopsis, or some other filamentous green alga. Others have suggested Padina, a brown alga which is highly abundant at sites where we find slugs, but which seems unlikely because all other Elysia species eat green algae. Ulva is also present, so it is also a candidate.
Because of the way they feed, it’s hard to know if Elysia are eating something unless they have sucked all the sap out of a plant. In the wild that’s not likely. However, since they keep the chloroplasts, and the chloroplasts have their own DNA, we can extract the DNA from the slugs and compare it to candidate food algae.
The plan was to set up a small molecular lab at the field station, extract DNA, amplify the chloroplast sequence using polymerase chain reaction (PCR), and take the PCR product back to the states for sequencing.
Rather than working out the procedures in a dirty, hot garage 12 hours from nowhere, I talked a couple of my UMd students into testing the protocols and reagents here in Maryland. They spent much of spring semester extracting DNA from plants and slugs, and we had everything worked out using a closely related species, Elysia clarki, along with a couple of species of Bryopsis and Halimeda.

Here's our experimental subject, with a divot on her parapodium from where we sampled the tissue. If you ever want to knock a marine mollusc out, isotonic (400 mM) MgCl2 does the job.

-
From where I sit, your Ca and Mg seem at the high end, which makes me wonder if your specific gravity is also high. What is your salinity, and what are you using to test it? Just grasping at straws here.
-
A few other notes: For the moment, I am using a Mag7 as a return, and just delivering through 1/2" vinyl tube with T's and ball valves. Because the flow is low, most are drained to the sump through 1" bulkheads with strainers, no fancy overflows. I would drain each tank to the sump, rather than chaining them, because it will allow you to take tanks offline, and you will avoid the problem of the last tank in the chain getting all the detritus and none of the stuff you're dosing.
If I magically had the time, I would hard-plumb the drains to a single standpipe, and add a bigger sump. I had toyed with the idea of a header tank above the rest, so that I could regulate the flow better, but that is not in the cards in the near future. Maybe when we move to the new building in a few years.
-
Hi Forrest,
For the office slug system, I have a 20L, 15, 10 and "half-ten" (10X20X6) plumbed into a 10 gallon sump. It gives a lot of flexibility for trying out different flow patterns, substrates and lighting for the slugs and their food algae. Plumbing them all into the same sump simplifies dosing, heating and chilling. Will post pics if you want.

-
I wouldn't add anymore fish. 2 clowns in a 6 gallon (4gallon volume) tank is max in my opinion.
+1; except that I would say you are already over capacity.
-
I put this over my fuge, and am pulling pounds of chaeto and caulerpa out. Screw it into your clipon, and you're good to go.
-
First one looks like a clingfish. The ones I have had have been easy to keep, and eat anything. Downside was that they hid really well.
-
I am back, and Jason at Batfish Aquatics did a great job of keeping everything alive and happy. It was not a simple system, but he paid enough attention to prevent clogs or the ATO running out. One less thing to worry about when the preparations for Baja got progressively more complicated. Plus, the price was very reasonable.
Big shout out for the company!
-
Wow, what an interesting project! Maybe it's the geneticist in me that always likes to break things, but have you thought about blocking BMP's actions? Increased growth in the presence of BMP could indicate an important role in regeneration, but could simply be promoting growth. If you were to inhibit regeneration by treating with Noggin or Chordin, which antagonize BMP signaling, or a drug that blocks BMP's action, you might be able to get at the question of whether BMP is necessary for regeneration. Science is never done!
-
Forgot this thread was still around.
Are they reproducing successful?
At this point, I have raised one generation from egg to adult, but want to do it more consistently and reliably. I have been distracted with other aspects of the project for the past year or so, but am getting the pieces together. It all seems to come down to food. The adults need a constant supply of high-quality algae (mostly Bryopsis) to be in the mood to lay eggs, and the juveniles need top-quality algae (Bryopsis or maybe Derbesia) to begin feeding. It's doable, but not trivial. It should be a good species for aquaculture, because the larvae do not need to feed in the planktonic (veliger) stage.
-
That is so true. I am constantly looking for the pdf.
Bruce
You may laugh now, but these days I usually find it easier to find my papers online than on my hard drive

-
Congrats! Now you'll always have a place where you can find it!
-
Zygote2k works for Tanks for your Business. I think he does still, at least. I have worked with them once on getting the tank set up in our daycare and they may be able to give you a price on a few trips to your office. They have people up here in Maryland that do service.
Joshua at Batfish Aquatics can help. He's in Silver Spring.
http://www.batfishaquatics.com
batfishaquatics@gmail.com
+1, Rob came and plumbed my new DT in Rockville and as Alan said, he has a colleague as well in the area.
I guess one concern would be would access, not sure how secure your building is and what the protocol would be to have someone unknown come in.
Will touch base with Rob and Batfish, thanks.
USG is an open campus, and parking is free at the moment. We have a security guard at the door, but a friendly "hello" will get you into the building. I can introduce whoever does the work to the small cohort at our end of the floor. Aside from student records, which will be locked up, there is nothing valuable or sensitive in my office.
-
In late June, I will be going away for three weeks, two of which I will have little or no connectivity. My wife will be home to watch those tanks, and I have a neighbor who is a WAMAS member who can help out.
The problem is the slug system in my office in Rockville. It consists of four tanks plumbed to a sump, used for growing slugs and algae. It is run by an Apex, which allows remote monitoring and control. The problem, though, is that the field station in Baja has very limited bandwidth, with frequent outages. Plus, the ATO will need to be filled, and sponges will need to be squeezed out every week or so to keep flow going. The plumbing is not very elaborate, but working with it would require experience with multi-tank systems.
I am just starting to get serious about this, so I am looking for recommendations for a service that works up here or an experienced member who would be willing to help out in exchange for cash, frags, or labor.
Here's what it looked like about six months ago. The only change is the addition of LED lights to all tanks.

-
I needed to occupy myself for a few minutes last night, so I made a GIF from some photos my wife took an the last trip. Imperfect, but amused me.

-
we just plan on doing softies nothing too fancy maybe a zoa garden
Sounds like a good plan. If you will be away, and don't plan on a reactor, then SPS would be more hassle than it's worth. You might consider some of the prettier macroalgae as well, like Botryocladia. Add a little variety and soak up a small amount of nutrients. My personal favorites for tanks that size are the little elacatinus gobies. I wish ORA would start breeding greenbanded gobies again, but you can easily get them wild caught.
A little mantis would also be very cool. A smasher, like N. wennerae, would be fun to watch and leave the corals alone. Snails and other inverts would not be safe, though.
-
I am also looking into a waterproof socket that I could just hang from the top of the sump, but I am having trouble finding one on Amazon. I will drive to a Home Depot to see if they have either in store.
Probably too late, but I used one like this http://www.homedepot.com/p/Commercial-Electric-Weatherproof-Socket-Black-R60-00055-000/100356874. I would strongly suggest soldering the connection to the cord and covering it with shrink tubing, rather than just using wire nuts.
-
I have some bryopsis rocks I can bring you. Are you around Thursday during the day?
I have some flexibility. Sending PM.




Hitchhiker: should I worry?
in General Discussion
A few corals rode in with some Bryopsis that DCReefer generously gave me. What species are they? Should I worry that they will take over my tank? Are they pests?
A colony on the rocks:
Here are a few frags in the overflow/frag area