-
Posts
270 -
Joined
-
Last visited
Content Type
Profiles
Forums
Gallery
Events
Store
Posts posted by Zaphodent
-
-
Hey guys,
Attached is a rough sketch of the house. Also a picture of the beam and joist on the opposite end of the house. Where the tank will be has drywall underneath so can't see.
How large of a tank can I place on the second floor of the house with a steel beam running underneath it? The joist unfortunately runs parallel with the tank location. I know there are a lot of other factors but looking for a rough estimate.
60 inch long tank is what we are looking at.
-
I like the SLW, they have a WiFi version now
Sent from my iPhone using Tapatalk -
So how are things going with blue clove polyps?
Sent from my iPhone using Tapatalk
Took me a while to find time to do this but it worked, cloves and even Aiptasisa died.
I did lose my turbo snails after a while and of course the GSP didn’t make it either. LPS and zoa weren’t affected much.
Sent from my iPhone using Tapatalk -
Try locking one clown in the main display so they both can see each other. Release after a few days and see what happens. I locked up both of my clowns that way when they got aggressive with some of my other fish, after they got out of jail, they were much calmer. Dunno if it will work in this case though, clown on clown aggression
Sent from my iPhone using Tapatalk -
Has anyone tried fenbendazole on eliminating blue clove polys? Any tips and suggestions with that or any alternative treatment tried?
Thanks

Sent from my iPhone using Tapatalk
-
Sorry didn’t fully read your message, 100 gallon sump, yikes, DIY like you said would be better

Sent from my iPhone using Tapatalk -
Look up Jax racks on eBay and he can customize to your specifications. I removed all my glass baffles and just updated mine with adjustable water height
Sent from my iPhone using Tapatalk -
I live in falls church (1 mile from inova)....can drop your water sample and I’ll test them, just leave me a snickers bar (joking)
Sent from my iPhone using Tapatalk -
A lot of different approach but also best to take out the big ones with kalk/water mix and let the shrimps/fish take care of it too.
Sent from my iPhone using Tapatalk -
Peppermint shrimp that eats aptasia?
Sent from my iPhone using Tapatalk -
Yes the tubing coming out of the T should be the highest point. So when the pump stop, it will suck air instead of water from the reactor.
Awesome, can’t wait to try that, just found a old thread on this after some digging
Sent from my iPhone using Tapatalk -
Install a T connector. ATO to T. One end of T goes to reactor. The other end points up, of course add some tubing to that end and hang up high above the reactor.
I do that with the output side of the reactor as well.
So one end for the ato, the other end goes the reactor. Sorry, kind of don’t get where the tubing for the third one will go?
Your saying to hang the T junction high above the reactor?
Thanks
Sent from my iPhone using Tapatalk -
Just got a avast kalk stirrer but it’s back siphoning into the ato reservoir. Installed a check valve but it’s creates too much resistance for ato to pump into the kalk stirrer.
Anyone have this issue with a kalk stirrer?
Sent from my iPhone using Tapatalk
-
In a classroom, could a kid have put something in there....
Sent from my iPhone using Tapatalk -
You said you've used reeflux with success on bryopsis?
-A-a-ron
Fluconazole being the active ingredient, there’s a big thread on it via reef2reef forum
Sent from my iPhone using Tapatalk -
I’ve used this on my main tank before, 70 gallon with success for bryopsis.
Just wanted to share before and after pic using fluconazole (aka reef flux) for GHA. 4-5 weeks in between pictures.
Took rocks out and manually removed all GHA as best as I could. Removed carbon. Move skimmer cup as high as possible and dialed it down.
Didn’t seem to irritate any corals.


Sent from my iPhone using Tapatalk
-
If you have a old router, you can connect the apex to it, then connect that router to your main hub.
I don’t think the usb one works, you’ll have to get an adapter that has Ethernet port.
Sent from my iPhone using Tapatalk
-
Pulsing Xenia?

Sent from my iPhone using Tapatalk
-



Sent from my iPhone using Tapatalk
-
While alkalinity typically doesnt affect pH in the aquarium to much degree, having it that low may exacerbate the delta between day and night provided your probe is actually correct, when was the last time you calibrated it? From what I'm seeing online, RSCP seems to mix in the 12-13 dKh range so you may have some test error responsible for a portion of that low number as well; probably worth having a buddy or LFS check a sample if you dont have another kit to compare the results with. I got tired of trying to line up the meniscus of the sample at the line on the Hanna cuvette so now I just use a syringe and add 10ml (easier and likely more consistent sample to sample now)
I'm assuming you're dosing alkalinity and calcium equally with the B-Ionic? Just because they're consumed at equal rates doesn't mean they'll be dosed at equal rates. They could have started at ranges that differed from your target in the beginning as well. If your calcium levels are stable and where you want, AND your alkalinity is actually down in the weeds, increase the dose of your alkalinity to slowly bring it into the range you desire and then cut it back to maintain that level was you get there.
Also, what is your salinity?
Salinity is 1.025 and my pH probe is good. I calibrate by refractometer and probe every six months, thanks to digital reminder apps.
RSCP at 12-13 dKH, I imagine would also depend how much salt we're mixing to make a water batch. I do mine at 1.025 so 11 sounds about right?
I just increased the timer for the alkalinity dosing so gonna try to monitor that. Thanks for the feedback. I'm monitoring the magnesium as well. I haven't had to dose that since my ranges seem within reference values but RSCP is so high to begin with so wondering if that is affecting my dKH.
With low alkalinity, you're likely to see wider pH swings since there is less buffering capacity. You should see your pH swings narrow some once your alk is back to reasonable levels.
How did your alk get so low? It's low enough that I'd suggest a series of water changes to bring it back up. Salinity is an important question to answer, but it won't answer why you're so low right now (with corals looking fine).
What kind of corals do you have in this tank, anyway? Softies? LPS? SPS? (Most, if not all, SPS would probably not tolerate such low alk for any extended period of time.)
Not sure how it got that low, was surprised. I did dose calcium a bit higher to catch up at one point but wasn't monitoring dKH until I ordered more refill agent. But there is definitely a wide swing in pH. I have easy to care for SPS like birdnest and monti caps but their big enough to consume a decent about of calcium. LPS and softies also in the tank. I have a hammer the size of a human head but it seems to be doing fine.
-
My Hanna checker shows an alkalinity of 63 ppm which comes out to 3.5 dKH. I tested it 2 days later just to make sure the trend was within the same area, 76 ppm which is a 4.2 dKH.
Corals are doing fine from appearance and not sure how low my dKH has been since I've never really tested for it besides calcium. I started dosing a few months ago after moving to a monthly water change (instead of every 2 weeks, got tiring). My calcium at one point was less than 300. I have a few large corals that consume calcium.
My calcium today is around 450, magnesium 1250. I dose B-ionic 2 part.
To get a baseline, I tested a fresh batch of water (red sea coral pro). dKH is 11 and magnesium was 1450-1500 (using red sea mg test kit). The high magnesium is normal with red sea coral pro from what I remember.
My pH is normal but does have a wide swing. Ranges between 7.8 to 8.4 from night to day, see pic attached.
Any thoughts on why my dKH is low?
-
Spray down with bottle of vinegar/water ? I do that with with mine once in a while
Sent from my iPhone using Tapatalk
-
Biopellets can make cyano worst. I gave up on biopellets, few have success

Sent from my iPhone using Tapatalk
-
Doesn't look like flatworms to me




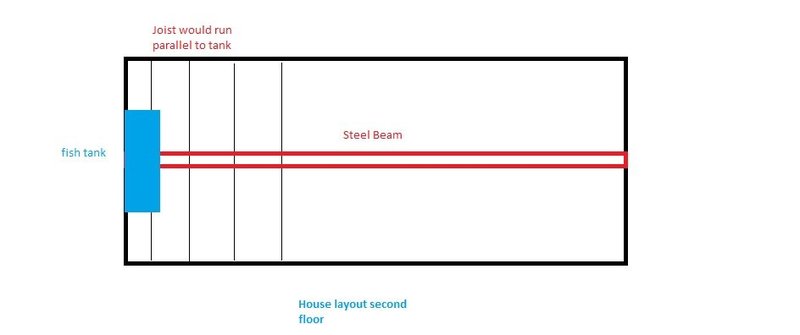


How large of a tank can I place on the second floor of house?
in General Discussion
It’s just one steel beam(used Microsoft paint haha)....it will be near the wall perimeter, maybe a few inches away from wall.
Footprint of tank will probably be 24 inches width wise so maybe will cover two joist.
Sent from my iPhone using Tapatalk