-
Posts
21,554 -
Joined
-
Last visited
Content Type
Profiles
Forums
Gallery
Events
Store
Everything posted by Origami
-
Next meeting is slated for North Bethesda Middle School and will feature Joe Yaiullo from the Long Island Aquarium. Sent from my Note 10+5g using Tapatalk
-

Getting Out & Selling 240G Reef Tank -Livestock First
Origami replied to loknar28's topic in General Discussion
Approved getting out of the hobby thread. -
Welcome back! It's been awhile. Sent from my Note 10+5g using Tapatalk
-
Hey Jon. Sorry. Been in a similar, heartbreaking place. Picking yourself back up can be tough, but live, learn and move forward. Good luck and my best to you and Maureen. Sent from my Note 10+5g using Tapatalk
-
Regarding the sump debris: Routine scheduled cleanup is pretty normal. Some people will shut the flow down and use an inexpensive bucket-head wet-or-dry vacuum (available at Home Depot) to suck the final bit of wet muck out of the bottom of their sump. They're about $30 these days.
-
We're pleased to announce our newest Platinum Sponsor, Capital Coral, to the WAMAS family! Unique in the sense that the founder of Capital Corals, Dr. Michael Gerdes, is also a co-founder of WAMAS (and a recent speaker of ours), this is truly a full-circle moment. Located in Albany, New York, Capital Corals was established to support ongoing coral research and restoration efforts. Their mission (as described on their website) is to work with the Ecotourism industry to implement the Reef Experience Snorkel Trail for local water beautification, coral education, and reef protection. They apply the most recent scientific advances and innovative approaches for coral reef habitat creation and restoration, working with local stakeholders to contribute to reef resilience and ecosystem services. They also provide research services for the scientific community to advance our understanding of coral reefs and promote their conservation. They maintain a coral aquaculture facility and wholesale store, as well, which we may use as a future source for WAMAS FragFest. Stay tuned!
-
@liquidgsr, just a suggestion, but post your questions in a separate thread in the General Discussion area rather than burying it in an old thread of yours. It may get more attention that way. Inverts (such as your shrimp) are very sensitive to salinity changes (much more so than fish) and need to be acclimated very, very slowly. Improper acclimation can lead to inverts dying, often times, within several days to a week or more, later. Have you figured out your other technical issues? If not, go ahead and post separately in the appropriate forum.
-

Welcome Capital Exotic Fish - WAMAS Sponsor!
Origami replied to Origami's topic in Capital Exotic Fish
Hey, Garrett! It's been a long time. Welcome back to the hobby! Sent from my Note 10+5g using Tapatalk -

Welcome Capital Exotic Fish - WAMAS Sponsor!
Origami replied to Origami's topic in Capital Exotic Fish
-
I'd like to take this opportunity to welcome Capital Exotic Fish to the WAMAS family as a Platinum Sponsor! Having opened their store in February 2023, they're celebrating their one year anniversary in DC, and have already made a splash at out most recent meeting with a big donation of quality, high-end corals right out of their newly launched saltwater department. (Thank you!) Chris and Sean are in charge of the day-to-day operations at Capital Exotic Fish. Both are experienced reefers and are excited about developing relationships with our reefing community. Chris comes from the AZA (Association of Zoos and Aquariums) side where he worked and maintained several reef exhibits for different intuitions and Sean has a lot of experience as a hobbyist as well. They carry a lot of new, exciting products such as Dalua lights and Quantum trace elements, along with a wide variety of corals from green star polyps to delicate SPS and chalices (and always have 100's of frags to choose from). On the fish side, they primarily keep a large inventory of inverts and nano fish but are willing to make special orders for whatever you are looking for. They are also excited to be the DC area source for UNS aquariums and their new reef aquariums. Located right down the road from the National Cathedral at 3404 Idaho Avenue, NW, they're literally centrally located for the entire WAMAS/DC community! Come by and see why so many people have already chosen their store for all their aquarium needs.
-
Yes, it is! Sent from my Note 10+5g using Tapatalk
-
Looks great, John. I'm looking forward to seeing you at the meeting. Sent from my Note 10+5g using Tapatalk
-

Sexy Shrimp (Thor amboinensis) breeding method and refinement
Origami replied to DaJMasta's topic in Propagation and Breeding
Great job! Please keep us updated on your methods and progress! -
Hard to say from that angle. Could be a baby. Watch it for a week or two.you may see tentacle buds. I had something similar many years ago with a GBTA and it turned out to be a baby, rather than the traditional split into a larger, more mature BTA. Sent from my Note 10+5g using Tapatalk
-

how long to keep a powder blue tang in jail?
Origami replied to astroboy's topic in General Discussion
It's not so much the size of the tank that concerns me, but the fact that they're both Acanthurus species and, therefore, aggression between the two will be more pronounced than between other tang species. If you can physically subdivide the tank for a couple of weeks to give the new inhabitant time to see the Powder Blue and to exchange chemistry (scent) with the other inhabitants, you may reduce the aggression. (I recall Copps doing something similar when introducing a new conspecific marine angel into one of his old (much smaller) tanks.) Otherwise, you may have to arrange or add some rockwork to break sight-lines and provide cover for the newcomer who's more likely to be bullied, even to the point of death. -
Here's an old thread that might be worth taking a look at, @liquidgsr, too.
-
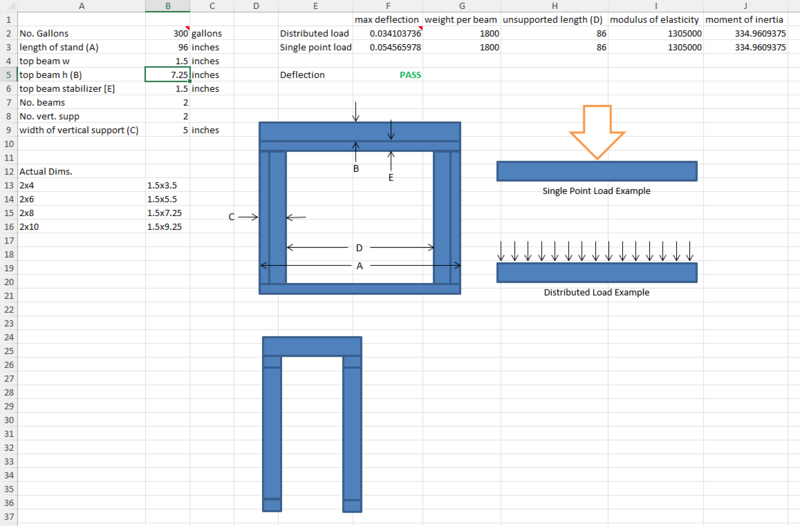
Brobak, this is snapshot of a beam deflection calculator that I used in the past to design my in-wall tank setup over ten years ago. Its purpose was to validate the beam deflection and therefore beam size needed to meet my goal of having two posts (one at either end of the beam to maximize access underneath the tank. First, I want to point out that my beam construction actually uses a main beam plus a 2x4 oriented horizontally to stabilize the beam from horizontal warping. You'll see that in the drawing. I'll also note that I addeda 3/4" plywood top, not shown. The legs in my (72") stand are constructed of two 2x4's arranged perpendicularly along their long edge and mounted to a 2x4 baseplate fastened to the concrete floor. The front of the stand is fastened into the wall studs to provide stability against racking. My 220 gallon tank sits 41 inches off the ground with this design using oversized 2x6's as the main beam. I've run the numbers for this design extended to your 96" - 300 gallon tank and it'll work with 2x6 beams, but I'd recommend that you go with the 2x8 for the added margin if you choose to go this route. Also, for safety's sake, given the long legs, I'd upgrade the top beam stabilizer, base plate, and legs to 2x6's. The calculation shows the beam deflection under the distributed load of the tank (estimated at 3600 pounds total) should be less than 1/20" using this design. Hope this isn't too late and that it helps.
-
You'll be fine with the configuration you describe. Sent from my Note 10+5g using Tapatalk
-
Welcome. I hope that you find your stay here rewarding. It sounds like you took your time cycling your tank. That's good. It's hard to say why your Montipora is suffering. Guessing about iodine or calcium deficiency, and then acting on that isn't a good idea, though. You really need to test. I suggest five core tests to start: First: Salinity. Get a refractometer, calibrate it, and measure your salinity. Second: Temperature. Next: Calcium and Alkalinity are two of the most important ions to measure. And finally, measure your magnesium. Note that I didn't include phosphate, nitrate, potassium, or anything else. Not that these are without value, but because these are the most basic water parameters to track. Test using quality test kits and post what you've found. Regarding your wrasse, how long did you have it before it died? Did you follow any sort of quarantine protocol before adding it to your tank? Sent from my Note 10+5g using Tapatalk
-
Confirm it looks like cyanobacteria. Is it less obvious just before lights turn on in the morning? Cyano can photosynthesize, so it tends to get worse as the day progresses. Sent from my Note 10+5g using Tapatalk
-
Ha, yes. An old thread, indeed. Sent from my Note 10+5g using Tapatalk
-

c02 scrubber, skimmer, and Milwaukee MC122
Origami replied to BowieReefer84's topic in General Discussion
I pull outside air into my skimmer as I run a calcium reactor. No CO2 scrubber, though. Has worked fine this way for over 10 years.. Sent from my Note 10+5g using Tapatalk -
Sorry to hear this, Lynn. I hope all works out for the best. You've got my number if you need it. Sent from my Note 10+5g using Tapatalk